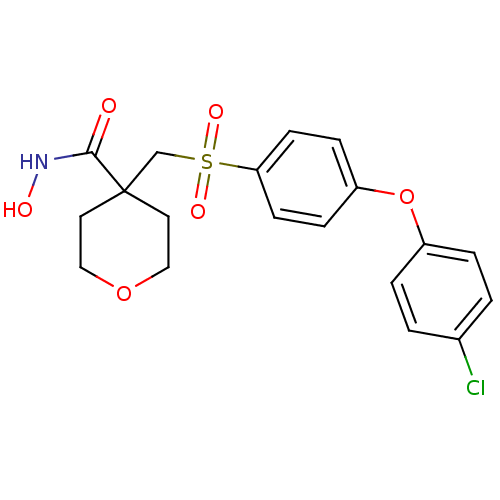
BDBM11863 4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N-hydroxyoxane-4-carboxamide::CHEMBL440498::RS 130830::RS-130,830::alpha-tetrahydropyran beta-sulfone 1B
SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1
InChI Key InChIKey=ROSNVSQTEGHUKU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 11863
Found 9 hits for monomerid = 11863
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 590nMAssay Description:Inhibition of matrix metalloprotease-1More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 590nMAssay Description:Inhibition of matrix metalloprotease-1 (MMP-1).Checked by AuthorMore data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 800nM ΔG°: -8.31kcal/molepH: 7.5 T: 2°CAssay Description:Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ...More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of human recombinant MMP1 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate after 40 mins by spectrofluorimetryMore data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:50 percent inhibition of human Matrix metalloprotease-1 by the cleavage of fluorogenic peptide MCA-Pro-Leu-Gly-Leu-Dpa-ala-Arg-NH2More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 233nMAssay Description:In vitro inhibitory activity against matrix metalloprotease-1More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of APMA-activated recombinant human MMP-1 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m...More data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of human recombinant MMP1 using fluorescence peptide Cy3-PLGLK(Cy5Q)AR-NH2 substrate by fluorescence assayMore data for this Ligand-Target Pair
TargetInterstitial collagenase(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 356nMT: 2°CAssay Description:Test compounds were serially diluted in deep well plates, and an aliquot of the inhibitor solutions was transferred to a Biomek deep well plate conta...More data for this Ligand-Target Pair